HIGHLIGHTS
Breakthrough TIL Therapy for Advanced Melanoma Now Offered at Montefiore Einstein Comprehensive Cancer Center
Montefiore Einstein Comprehensive Cancer Center is among the first academic medical centers in the New York Metropolitan Area to offer tumor-infiltrating lymphocyte (TIL) therapy—the newly FDA-approved cellular immunotherapy treatment for advanced melanoma patients who showed no clinical improvement with first-line therapies.
"Nearly one-third of melanoma patients don't respond to traditional treatments like immune checkpoint inhibitors," states Dr. Yvonne M. Saenger, Director of the Montefiore Einstein Comprehensive Cancer Center Immunotherapy Program and Associate Professor of Oncology, Pathology & Microbiology and Immunology at Albert Einstein College of Medicine. "For these patients, TIL therapy represents a groundbreaking second-line option, backed by clinical evidence."
Initially only available at the National Cancer Institute, Montefiore Einstein Comprehensive Cancer Center is now bringing TIL therapy to patients in the Bronx. The treatment marks the first FDA-approved cellular therapy for solid tumors, offering new possibilities for patients with limited therapeutic options. The team has been actively contributing research in cellular therapeutics, including early-stage clinical trials led by Dr. R. Alejandro Sica, Assistant Professor of Medicine at Albert Einstein College of Medicine, and basic science work led by Dr. Harris Goldstein, Associate Dean of Scientific Affairs at the Children's Hospital at Montefiore Einstein.
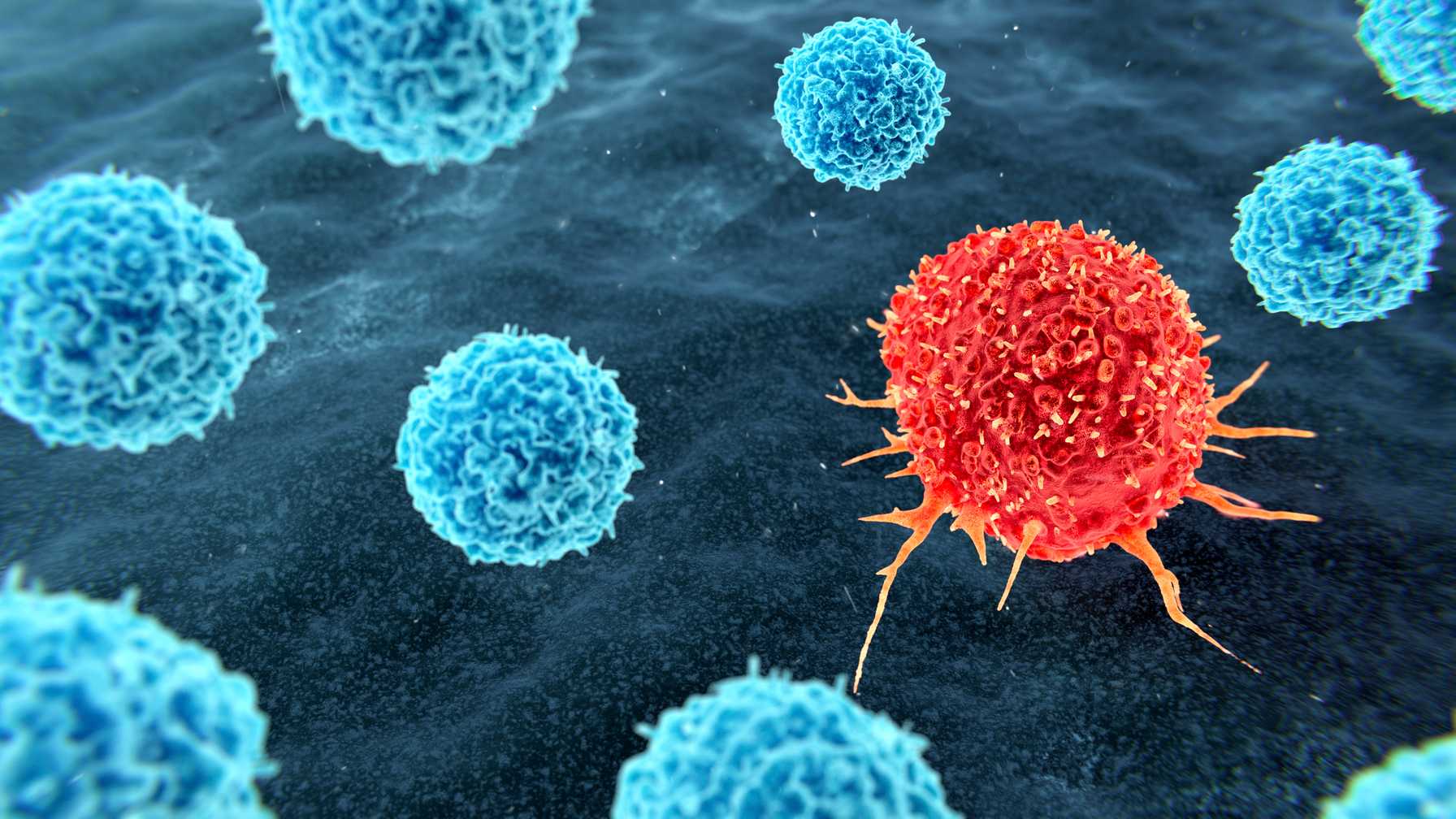
TIL therapy harnesses the patient's own immune system through a multi-step process. First, surgeons remove tumor tissue containing cancer-fighting T cells. Then, tumor-infiltrating lymphocytes are isolated, multiplied in a specialized lab, and activated. Patients undergo preparatory immunotherapy similar to that used for bone marrow transplantation. The TILs are then reinfused, ready to recognize and attack cancer cells.
While TIL therapy requires specialized facilities only available at major academic centers like Montefiore Einstein, the treatment model allows for collaboration with community oncologists. Patients visit Montefiore Einstein for surgery and cell extraction, receive therapy there, and return to their local oncologists for follow-up care. In clinical trials, approximately one-third of patients had meaningful tumor responses—some experiencing complete responses lasting years after a single treatment.
Montefiore Einstein also offers novel oncolytic viral therapies for patients who are too frail for TIL therapy. This FDA-approved option is available as standard of care through clinical trials. Dr. Saenger has extensive experience in this approach.
Community oncology practices interested in referring melanoma patients are encouraged to contact the Montefiore Einstein TIL therapy program. Our multidisciplinary team works closely with referring physicians, ensuring seamless coordination and communication.
For patient referrals, please call Yvonne Saenger, MD at 646-425-5734.
Patient referrals
At Montefiore Einstein Comprehensive Cancer Center, we know that providing patients with the best possible oncology care includes teamwork and trust. We work closely with our valued referring physicians to ensure open communication and reliable expertise.
Contact us
Anne McDarby
Associate Director, Network Program Management
Montefiore Einstein Comprehensive Cancer Center
1-718-862-8840 /ext. 5034
amcdarby@montefiore.org
Andrea Peirce
Assistant Director, Communications
Montefiore Einstein Comprehensive Cancer Center
andrea.peirce@einsteinmed.edu